EU approves higher daily intake limits for Zooca® Calanus Oil
Following our application in December 2024, the European Commission has approved updated conditions for the use of Zooca® Calanus Oil under the Novel Food regulation (Commission Implementing Regulation (EU) 2025/1513).
The amendment introduces higher astaxanthin levels, higher daily intake limits, and more flexible labelling rules hence strengthening the regulatory framework for Zooca® Calanus Oil in Europe.
What’s new for adults — our primary supplement audience
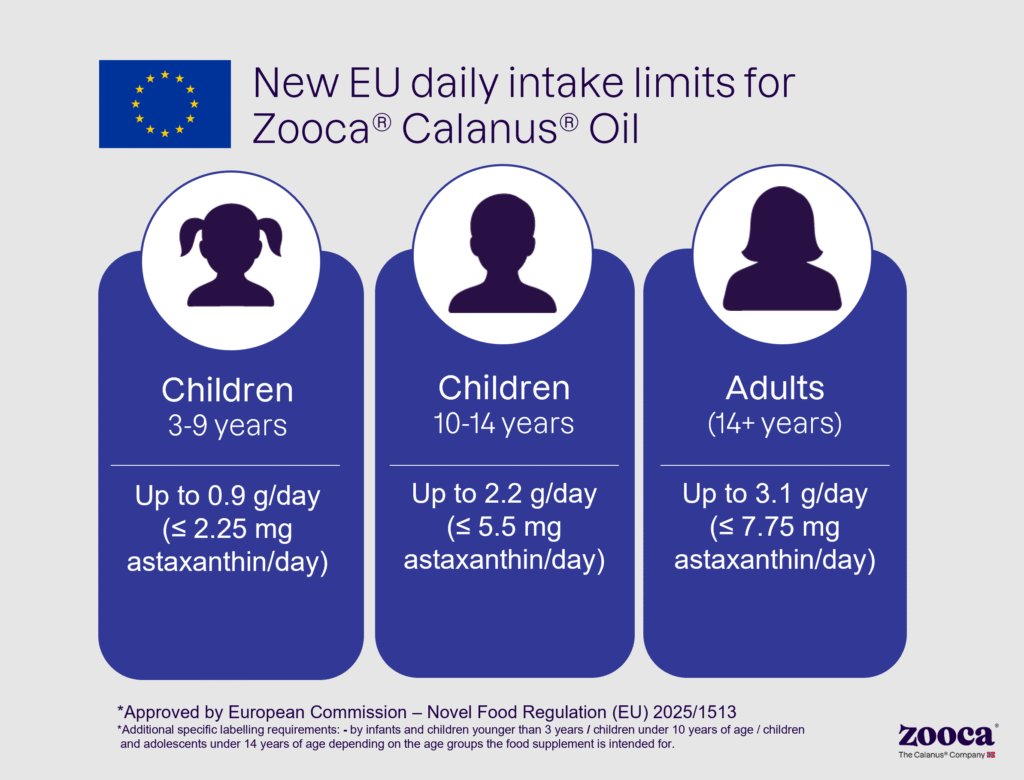
Dietary supplements are most commonly consumed by adults, who represent the largest and most commercially significant user group. The revised Novel Food conditions are therefore particularly important for this segment. With the new maximum daily intake for adults raised from 2.3 g to 3.1 g/day (≤ 7.75 mg astaxanthin/day), brand owners can offer formulations that deliver more of Zooca® Calanus Oil’s key bioactive components. Zooca® Calanus Oil has a unique 3-in-1 natural combination of over 40 different marine fatty acids, including the well-known omega3s, in addition to marine policosanols, and astaxanthin. This combination supports EFSA-approved health claims for EPA and DHA and aligns with consumer demands. The updated intake limits open new possibilities for more potent and flexible formulations.
Key changes
- Higher astaxanthin limits: ≤ 0.25% (max 2500 mg/kg) for all age groups.
- Updated daily intakes:
- Adults (14+ years): up to 3.1 g/day (< 7.75 mg astaxanthin/day)
- Children 10–13 years: up to 2.2 g/day (< 5.5 mg astaxanthin/day)
- Children 3–9 years: up to 0.9 g/day (≤ 2.25 mg astaxanthin/day)
- Simplified labelling rules: Products must state which age group they are not intended for and carry an advisory against same-day use with other astaxanthin-containing supplements.
Examples:- If intended for individuals aged 10+, state: Not to be consumed by children under 10 years.
- If intended for individuals aged 14+, state: Not to be consumed by children and adolescents under 14 years.
- If intended for individuals aged 3+, state: Not to be consumed by infants and children under 3 years.
This link provides information in the following languages: BG, ES, CS, DA, DE, ET, EL, EN, FR, GA, HR, IT, LV, LT, HU, MT, NL, PL, PT, RO, SK, SL, FI, SV.
Why is this important?
This regulatory change provides greater flexibility in supplement design. It broadens the possible target groups and makes it easier to create compliant products for children, adolescents, and adults with the same ingredient base. The change supports stronger use of existing EFSA-approved claims for EPA and DHA.
We are proud to have initiated and secured this positive change, further strengthening the regulatory foundation for Zooca® Calanus Oil in the EU market.